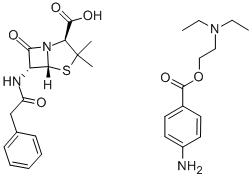
产品详情;
CasNo: 54-35-3
分子式: C29H38N4O6S
外观: colourless viscous liquid
交货时间: In stock
生产能力: 10000|Metric Ton|Month
技术指标: 99%
| Procaine penicillin G Basic information |
| Product Name: | Procaine penicillin G |
| Synonyms: | BENZYLPENICILLIN PROCAIN SALT;PROCAINE BENZYLPENICILLIN;PROCAINE PENICILLIN G;PENICILLIN G PROCAINE;PENICILLIN-G PROCAIN SALT;Procaine penicillin G(1% lecithin/citrate acid);Penicillin G procaine +1% lecithin CP200/BP/USP/EP;Procain Penicillin G |
| CAS: | 54-35-3 |
| MF: | C29H38N4O6S |
| MW: | 570.7 |
| EINECS: | 200-205-7 |
| Product Categories: | PHARMACEUTICALS |
| Mol File: | 54-35-3.mol |
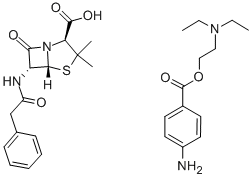 |
|
| Procaine penicillin G Chemical Properties |
| Melting point | 129-130 °C |
| density | 1.1335 (rough estimate) |
| refractive index | 1.6000 (estimate) |
| CAS DataBase Reference | 54-35-3 |
| EPA Substance Registry System | 4-Thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid, 3,3-dimethyl-7-oxo-6-[(phenylacetyl)amino]- (2S,5R,6R)-, compd. with 2-(diethylamino)ethyl 4-aminobenzoate (1:1) (54-35-3) |
| Safety Information |
| Toxicity | LD50 orl-rat: >2 g/kg ABANAE 3,534,55/56 |
| MSDS Information |
| Procaine penicillin G Usage And Synthesis |
| Chemical Properties | White, fine crystals or powder; odorless; relatively stable to air and light; solutions dextroro- tatory. Sparingly soluble in water; slightly soluble in alcohol; fairly soluble in chloroform. |
| Originator | Duracillin,Lilly,US,1948 |
| Uses | Antibacterial. |
| Manufacturing Process | There was added to 250 ml of a concentrated butyl acetate extract containing 74,000 units of the acid form of penicillin per ml, 50 ml of a butyl acetate solution containing 0.238 g per ml of procaine base. The solution was agitated for one hour. The precipitate which formed was very gummy and not in the form of discrete crystals. This precipitate was crystallized by scratching the side of the vessel and agitating further. After this treatment 18.25 g of crystalline procaine penicillin was obtained which assayed 1010 units per mg representing a yield of 99.6% of the activity contained in the concentrated extract. |
| Brand name | Duracillin(Lilly); Pfizerpen (Pfizer). |
| Therapeutic Function | Antibacterial |
| General Description | The first widely used amine salt of penicillin G wasmade with procaine. Penicillin G procaine (Crysticillin,Duracillin, Wycillin) can be made readily from penicillin Gsodium by treatment with procaine hydrochloride. This saltis considerably less soluble in water than the alkali metalsalts, requiring about 250 mL to dissolve 1 g. Free penicillinis released only as the compound dissolves and dissociates.It has an activity of 1,009 units/mg. A large number ofpreparations for injection of penicillin G procaine are commerciallyavailable. Most of these are either suspensions inwater to which a suitable dispersing or suspending agent, a buffer, and a preservative have been added or suspensions inpeanut oil or sesame oil that have been gelled by theaddition of 2% aluminum monostearate. Some commercialproducts are mixtures of penicillin G potassium or sodiumwith penicillin G procaine; the water-soluble salt providesrapid development of a high plasma concentration of penicillin,and the insoluble salt prolongs the duration of effect. |
| Safety Profile | Moderately toxic by intraperitoneal and intramuscular routes. Slightly toxic by ingestion and subcutaneous routes. When heated to decomposition it emits toxic vapors of NOx, and SOx. |
相关产品
-
2-氨基-1,4-苯二磺酸一钠
CAS:24605-36-5
-
反-4-辛烯
CAS:14850-23-8
-
2-(9,9'-螺双[9H-芴]-2-基)-4,6-双([1,1':3',1''-三联苯]-5'-基)-1,3,5-三嗪
CAS:1233200-52-6





